Beyond the Itch: The 2025 Guide to New Eczema Treatments & Cures
You know the feeling. It starts as a subtle tingle, then builds into a burning fire that no amount of willpower can suppress. You wake up at 3:00 AM, sheets bloodied, skin raw, wondering if this cycle will ever end. For years, the medical advice I’ve seen dispensed to patients was frustratingly simple: moisturize often and apply topical steroids when it flares. It felt like putting a bandage on a broken bone.
But the landscape has shifted dramatically. As we moved through late 2024 and settled into 2025, the FDA approved six groundbreaking treatments that are not just “new versions of old drugs”—they are entirely new mechanisms of action. We have moved from simply suppressing inflammation to precision medicine that targets the specific proteins causing your itch.
Americans currently living with some form of eczema.
Global prevalence of eczema in children.
According to the National Eczema Association, eczema affects approximately 10% to 20% of children and 2% to 10% of adults worldwide. Yet, until recently, options for severe cases were limited. This article is your definitive roadmap to the 2025 revolution in dermatology—from ending the “neuro-itch” to steroid-free creams for toddlers.
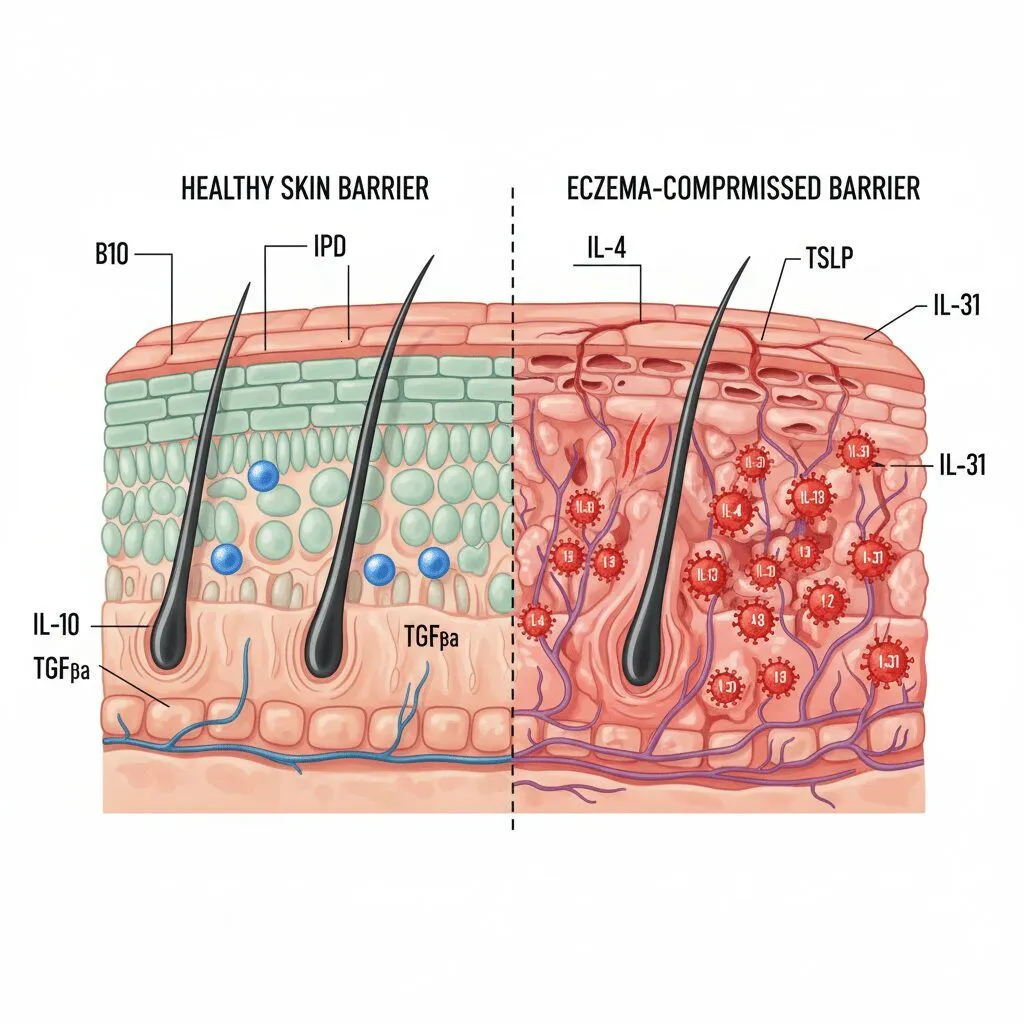
The “Itch” Revolution: Targeting IL-31
If you ask any eczema sufferer what the worst symptom is, they rarely say “the rash.” They say “the itch.” For decades, doctors treated the inflammation hoping the itch would subside. But in a major shift, science has now separated the two.
What is Nemluvio (nemolizumab)?
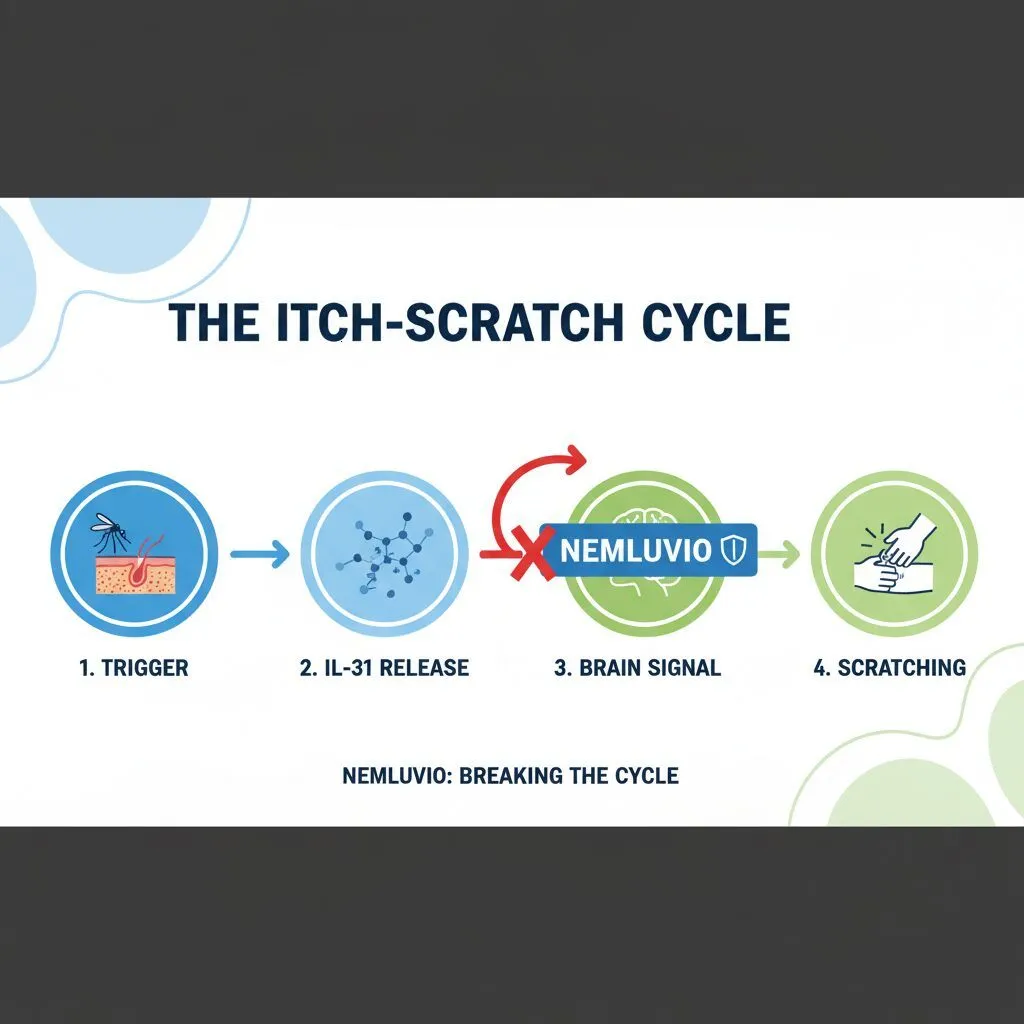
Approved by the FDA in December 2024, Nemluvio is the first biologic specifically designed to target the “itch cytokine” known as IL-31. While previous biologics like Dupixent targeted IL-4 and IL-13 (inflammation pathways), Nemluvio goes straight to the nerve signal that tells your brain to scratch.
The mechanism here is fascinating. IL-31 is often called the “master switch” for pruritus (itch). By blocking this receptor, the drug disrupts the neuro-itch signal. According to a press release from Galderma regarding the FDA approval on Dec 16, 2024, Nemluvio showed statistically significant reduction in itch as early as Week 1 in clinical trials.
This is a game-changer for adolescents (12+) and adults who find that even when their skin looks better, they can’t stop scratching. It essentially uncouples the sensation of itch from the physical state of the skin, breaking the damage cycle.
Who is it for?
Currently, this injection is approved for adolescents 12 years and older with moderate-to-severe atopic dermatitis (AD) whose disease is not adequately controlled with topical prescription therapies.
The New Era of Biologics: Less Frequent Dosing
For those of us who have followed the biologic market since 2017, the complaint has always been the frequency of injections (usually every two weeks). 2025 brings relief to “needle fatigue.”
Enter Ebglyss (lebrikizumab): The Monthly Option
Ebglyss (lebrikizumab) represents the next generation of IL-13 inhibitors. While it targets a similar pathway to previous drugs, its binding affinity is different, allowing for a dosing schedule that many patients find more manageable.
The standout feature of Ebglyss is the maintenance phase. After an initial induction period (loading doses), patients can transition to a once-monthly injection while maintaining skin clearance. For a teenager or a busy professional, cutting the number of annual injections in half is a significant lifestyle improvement.
According to the ADvocate 1 & 2 Trials (2024), which were pivotal for its approval, Ebglyss demonstrated high efficacy rates. The studies showed that a significant percentage of patients achieved EASI-75 (a 75% improvement in the Eczema Area and Severity Index) and maintained it through the monthly dosing schedule.
- Dupixent (dupilumab): Targets IL-4 and IL-13. Usually dosed every 2 weeks.
- Nemluvio (nemolizumab): Targets IL-31 (Itch).
- Ebglyss (lebrikizumab): Targets IL-13 specifically. Maintenance dose once every 4 weeks.
No More Steroids? The Rise of Advanced Topicals
One of the most common fears I hear from parents in the clinic is “steroid phobia”—the fear of skin thinning (atrophy) or Topical Steroid Withdrawal (TSW). The pharmaceutical industry has listened, and 2025 offers powerful non-steroidal options that are safe even for young children.
Zoryve (roflumilast) Cream: The PDE4 Inhibitor Breakthrough
Zoryve is a topical PDE4 inhibitor. Unlike steroids, which dampen the immune system broadly, PDE4 inhibitors target an enzyme specifically involved in inflammation. What makes Zoryve unique in 2025 is its vehicle (the cream base) and its expanded age approval.
According to data from the Arcutis Biotherapeutics INTEGUMENT-OLE Study released in October 2024, 71.9% of pediatric patients achieved a 75% improvement in EASI scores after 56 weeks. Critically, this cream does not contain propylene glycol (a common irritant) and doesn’t sting upon application—a massive victory for parents trying to apply cream to a screaming toddler.
Approval Timeline Key:
It was approved for children aged 6+ in July 2024, and subsequently for children as young as 2 to 5 years old in October 2024. If your pediatrician hasn’t mentioned this yet, it’s worth bringing up during your next visit.

Vtama (tapinarof) Cream: The “Remission” Cream
Vtama works via a completely novel pathway called an aryl hydrocarbon receptor agonist. It sounds complex, but here is what matters to you: Remission. Clinical trials have suggested that after using Vtama to clear the skin, patients can stop using the cream and maintain clear skin for a significantly longer period than with traditional steroids. This “drug holiday” potential is a major psychological relief for chronic sufferers.
Finally, Help for Hand Eczema: Anzupgo
Chronic Hand Eczema (CHE) is often grouped with general atopic dermatitis, but it is a distinct, debilitating beast. It ends careers. It makes shaking hands, typing, or cooking painful.
According to the National Eczema Association, the lifetime prevalence of chronic hand eczema is estimated at 14.5% of the general population. Despite this, treatments have been scarce until Anzupgo (delgocitinib).
Anzupgo is a topical JAK inhibitor specifically approved for this condition. Unlike oral JAK inhibitors (like Rinvoq), which carry boxed warnings for systemic side effects, Anzupgo is applied locally to the hands. It gained traction in mid-2025 as the go-to prescription for dyshidrotic and fissured hand eczema that resists steroids.
The Future Frontier: Microbiome & Gut Health
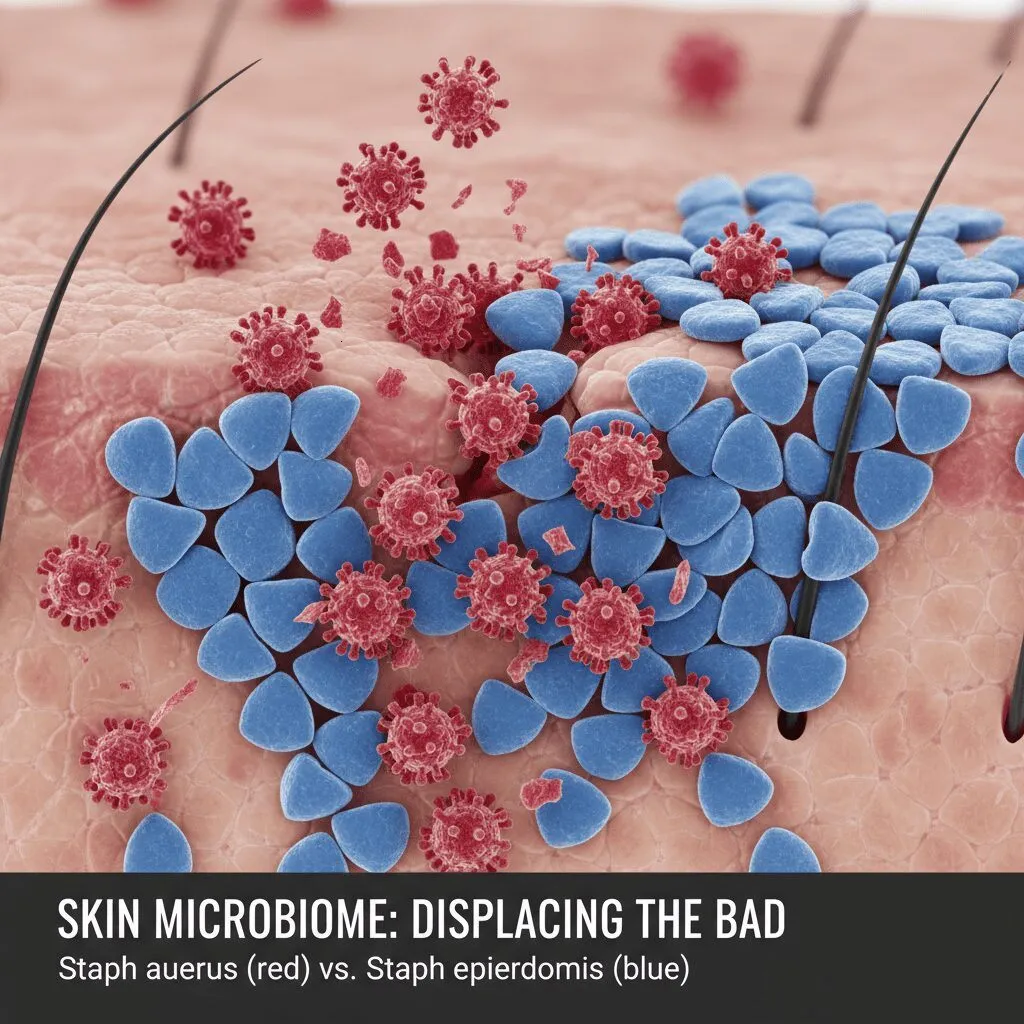
While drugs treat the symptoms, research in 2025 is finally closing in on the root cause: the microbiome. We’ve known about the “Gut-Skin Axis” for years, but the data is getting actionable.
The focus is on the war between Staphylococcus aureus (the bad bacteria that colonizes 90% of eczema skin) and Staphylococcus epidermidis (the good bacteria). 2025 research is moving away from broad antibiotics, which kill both, toward “precision bacteriotherapy.”
Recent insights from Fecal Microbiota Transplant (FMT) studies, such as the Shanghai & GPER 2024 studies, suggest that resetting the gut flora can lower systemic inflammation levels (IgE) in the blood. While this isn’t a pill you can buy at CVS yet, clinical trials for topical probiotic treatments are recruiting heavily in 2025.
Patient Roadmap: Which Treatment is Right for You?
With so many new approvals, the choice can be overwhelming. Based on the current clinical guidelines and FDA approvals, here is how the landscape looks for a patient in 2025.
| Drug Name | Type | Best For… | Key Benefit |
|---|---|---|---|
| Nemluvio | Injection (Biologic) | Severe Itch (Prurigo nodularis/AD) | Targets itch specifically (IL-31). |
| Ebglyss | Injection (Biologic) | Maintenance Therapy | Once-monthly dosing frequency. |
| Zoryve | Cream (PDE4) | Pediatric / Sensitive Areas | Steroid-free, approved for ages 2+. |
| Vtama | Cream (AhR Agonist) | Long-term Management | Potential for remission periods. |
| Anzupgo | Cream (Topical JAK) | Chronic Hand Eczema | Specific efficacy for thick hand skin. |
Frequently Asked Questions
What is the new eczema injection approved in 2024?
The primary new injection is Nemluvio (nemolizumab), approved in December 2024. It is distinct because it targets the IL-31 cytokine responsible for the itch signal, rather than just general inflammation.
Is Zoryve safe for toddlers with eczema?
Yes. As of October 2024, the FDA expanded approval for Zoryve (roflumilast) cream 0.15% to include children aged 2 to 5 years. Studies showed a favorable safety profile with no evidence of systemic side effects common with steroids.
How does Nemluvio work for eczema itch?
Dr. Jonathan Silverberg explains that Nemluvio addresses the “neuroimmune cytokine’s role.” Essentially, it blocks the reception of IL-31, preventing the nerve ending from sending the “scratch now” signal to the brain, even if some inflammation is still present.
Can diet cure eczema according to new research?
While diet is rarely a “cure-all,” 2025 research into the Gut-Skin Axis confirms that reducing systemic inflammation via diet and potentially Fecal Microbiota Transplants (FMT) can significantly improve symptoms, though it is best used as an adjunct to the therapies listed above.
Final Thoughts
The era of “just live with it” is over. We have moved from a scarcity of treatments to an abundance of precision tools. Whether you need to stop the itch (Nemluvio), simplify your injection schedule (Ebglyss), or treat your toddler without steroids (Zoryve), 2025 offers genuine hope.
Consult your dermatologist to see which of these new therapies aligns with your medical history.
